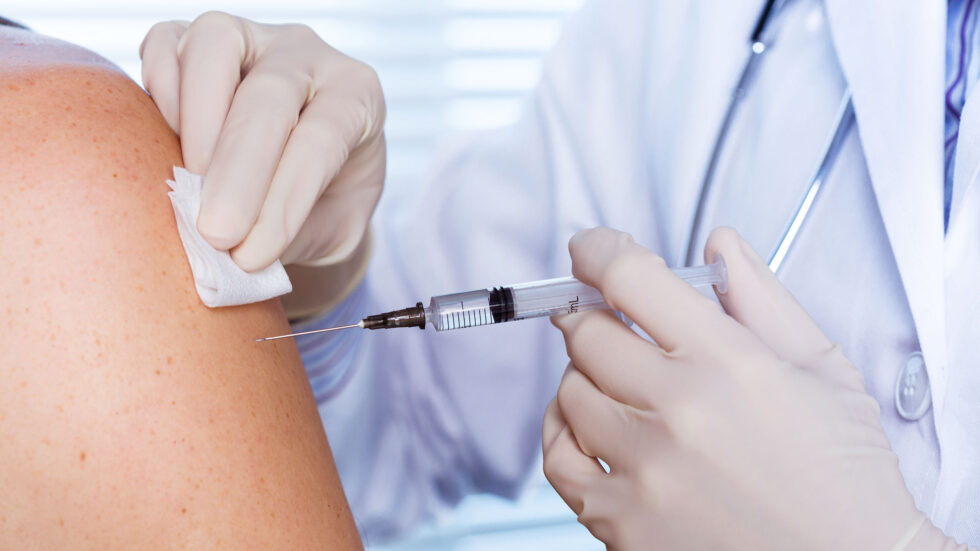
As researchers race to develop a COVID-19 vaccine, two Rutgers Global Health Institute core faculty members discuss how clinical trials work, the ethics of developing and distributing a vaccine, safety and efficacy in clinical trials, and what a successful vaccine may mean. Associate Professor Shobha Swaminathan is principal investigator at the Rutgers New Jersey Medical School Clinical Research Center and the medical director of Infectious Diseases Practice at University Hospital, and Michael K. Gusmano is a bioethicist and professor of health policy at the Rutgers School of Public Health. To read the full story.
Recent Posts
- To Heal Skin, Scientists Invent Living Bioelectronics.
- Register for Children’s Specialized Hospital Distinguished Lecture on 8/14
- Researchers Shed Light on Cause of ‘Happy Hypoxia’ in COVID-19 Patients.
- Upending Conventional Wisdom, Cannabis Use Doesn’t Hinder PTSD Therapy.
- Many Firearm Owners Can’t Recognize When a Cable Lock Is Properly Installed.
Categories
- Community (1,944)
- Covid (971)
- CTO Events (1)
- News (2,488)
- Pilots (20)