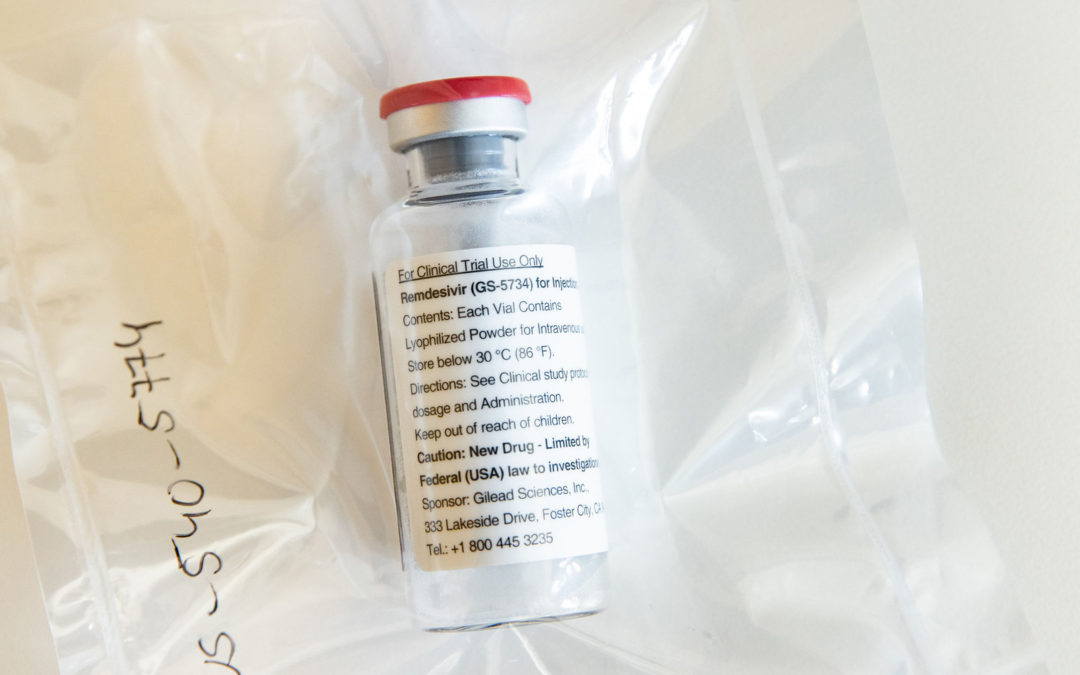
Now that the Food and Drug Administration has authorized remdesivir for emergency use in seriously ill COVID-19 patients, the experimental drug is another step closer to full approval. That’s when most drugs get price tags. Gilead Sciences, which makes remdesivir, is donating its initial supply of 1.5 million doses, but the company has signaled it will need to start charging for the drug to make production sustainable. It’s unclear when that decision might be made. “It’s hard to imagine a situation in which there will be more public scrutiny,” said Michael Carrier, a professor at Rutgers School of Law who specializes in antitrust and pharmaceuticals. “On the one hand, Gilead will try to recover its R&D in an atmosphere in which it is able to potentially make a lot of money. On the other hand, the pressure will be intense not to charge what’s viewed as too high a price.” To read the full story.
Recent Posts
- To Heal Skin, Scientists Invent Living Bioelectronics.
- Register for Children’s Specialized Hospital Distinguished Lecture on 8/14
- Researchers Shed Light on Cause of ‘Happy Hypoxia’ in COVID-19 Patients.
- Upending Conventional Wisdom, Cannabis Use Doesn’t Hinder PTSD Therapy.
- Many Firearm Owners Can’t Recognize When a Cable Lock Is Properly Installed.
Categories
- Community (1,944)
- Covid (971)
- CTO Events (1)
- News (2,488)
- Pilots (20)