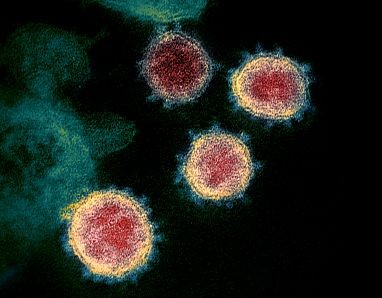
Evelo Biosciences, Inc. (Nasdaq:EVLO), Rutgers University, and Robert Wood Johnson University Hospital today announced the submission of an Investigational New Drug (IND) application for an Evelo-sponsored Phase 2 clinical study evaluating the safety and efficacy of EDP1815 for the treatment of hospitalized patients with newly diagnosed COVID-19. The study will be led by Reynold A. Panettieri, Jr., M.D., Vice Chancellor for Translational Medicine and Science at Rutgers Biomedical and Health Sciences and Professor of Medicine at Rutgers Robert Wood Johnson Medical School. To read the full story.
Home / News / Evelo Biosciences, Rutgers University, and Robert Wood Johnson University Hospital Announce Submission of IND for a Phase 2 Study of EDP1815 in COVID-19 Patients
Recent Posts
- To Heal Skin, Scientists Invent Living Bioelectronics.
- Register for Children’s Specialized Hospital Distinguished Lecture on 8/14
- Researchers Shed Light on Cause of ‘Happy Hypoxia’ in COVID-19 Patients.
- Upending Conventional Wisdom, Cannabis Use Doesn’t Hinder PTSD Therapy.
- Many Firearm Owners Can’t Recognize When a Cable Lock Is Properly Installed.
Categories
- Community (1,944)
- Covid (971)
- CTO Events (1)
- News (2,488)
- Pilots (20)