Scientific Review Board
What is a Scientific Review Board (SRB)?
The NJACTS Scientific Review Board supports investigator-initiated studies by reviewing non-oncology protocols to assess proper statistical design, enrollment goals, feasibility (including adequate research staffing, competing trials, funding, resources, and departmental support) to help accelerate IRB approval and to help ensure study success.
The degree of review required may vary based on the type of research, funding, and institutional factors involved.
The Scientific Review Board (SRB) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to answer that question.
The SRB review will primarily focus on the elements of good scientific study design. Proposals will be evaluated for the following criteria:
- clarity of the research question,
- appropriateness and efficiency of design,
- rigor and feasibility of methods,
- qualifications and expertise of the research team,
- scholarship and pertinence of background material and rationale,
- adequacy of sample size and relevance of controls,
- and the validity of the statistical analysis plan.
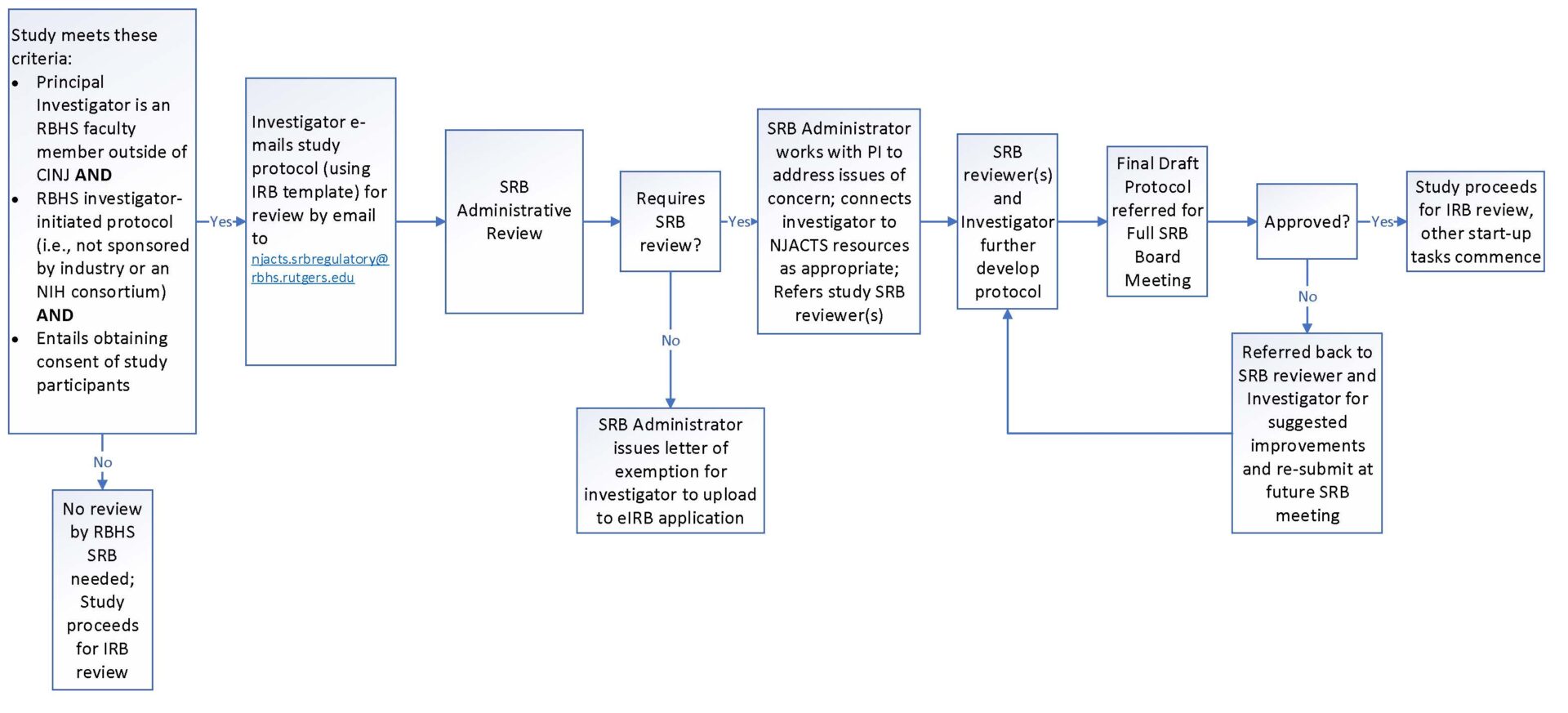
In addition, the SRB may comment on the proposal’s scientific relevance or compelling ethical or patient safety issues. The SRB Committee member(s) will reach out to the PI of the study and provide feedback prior to the scheduled SRB meeting. The SRB Committee member(s) will present a summary of their evaluation and final recommendation to the entire SRB Committee at the regularly scheduled monthly SRB meeting.
SRB review is a requirement for all RBHS PI-initiated studies that are greater than minimal risk and/or have a consent process in place for their studies. Once the SRB has reviewed and approved the protocol for scientific merit the PI can then submit to the IRB for full review of their submission. The IRB reserves the right to send any study to the Scientific Review Board for evaluation, especially in cases where the study has been tabled or disapproved by a fully convened IRB.
Please note, a member of the Scientific Review Board will not review any protocol in which s/he has an interest in the study.
Deadline for SRB Review
Study information intended for the Scientific Review Board must be submitted at least 10 business days prior to the desired review date to guarantee a timely response. Principal Investigators are encouraged to present their protocol at the SRB meeting for which they have submitted study documents. No meetings will be held on institutional holidays; meetings may need to be rescheduled in the absence of a quorum.
Criteria for SRB Review
The Scientific Review Board will review studies under the following review criteria:
- Objectives: Clearly stated specific aims aligned with well-defined endpoints and appropriate study design.
- Scientific Merit/Background and Rationale: Justification for conducting the study; results of similar or pilot data; current literature cited.
- Design: Clearly describes how stated objectives will be achieved, methods to acquire data, and strategies to overcome anticipated barriers. Addresses randomization, minimization of bias, patient follow-up, and blinding (if applicable).
- Eligibility Criteria: Specific inclusion/exclusion requirements and stratification factors (if applicable).
- Outcome Characteristics and Endpoint Definitions: Clearly defined primary and secondary endpoints/outcomes.
- Statistical Analysis and Sample Size: Appropriate and adequate study design statistical analysis plan. Prospective analysis plan, including sample size justification to achieve study objectives and plans to minimize missing data.
- Data Management: Practices and procedures in order to manage data analysis, quality, cleaning, and storage.
- Principal Investigator and Study Site Qualifications and Resources: Has the necessary skills, experience, time, and resources to ensure that the study can be successfully completed, including identification of personnel to provide statistical computations and statistical expertise. A plan to register protocol with clinicaltrials.gov, if applicable.
If the SRB Committee member(s) has found that each criterion is Present/Acceptable, the protocol will be presented at a fully convened SRB meeting for final discussion and approval. If any criteria are absent or deemed not acceptable, the SRB Committee Member(s) will return comments to the Principal Investigator (PI) for revisions. The PI must address all comments by making recommended revisions or providing further justification for resubmission. The PI must respond point-by-point to comments and revise the protocol accordingly. Once the committee member(s) accepts the revised protocol, it will notify the PI and the protocol will be presented at the SRB meeting for final approval.
The SRB will issue the PI an approval notice to be submitted with their IRB submission.
Additional Information for Researchers
Differences between clinical trials and clinical studies using the following four questions:
- Does the study involve human subjects?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If the answer to all four questions is “yes,” then the clinical study would be considered a clinical trial according to the NIH definition.
Note that if the answers to the 4 questions are yes, the study meets the NIH definition of a clinical trial, even if…
- You are studying healthy participants
- Your study does not have a comparison group (e.g., placebo or control)
- Your study is only designed to assess the pharmacokinetics, safety, and/or maximum tolerated dose of an investigational drug
- Your study is utilizing a behavioral intervention
- Studies that involve secondary research with biological specimens or health information, or studies that are intended solely to refine measures are not considered clinical trials.
Scientific Review Board Process Map