eReg Training & Access
Because eReg is a 21 CFR Part 11 complaint product, all users are required to complete the web-based training from Advarra University and then submit two forms to the RBHS Clinical Trials Office.
For Monitor Access:
If this is your first time using eReg for monitoring, please follow the “Monitors Without An Advarra University Account” pathway. If you have previously used eReg for Rutgers or for another institution, follow the instructions titled “Monitors With An Advarra University Account”.
Monitors Without An Advarra University Account
Step 1: Guest NetID
All monitors are required to have a guest NetID in order to access eReg, due to University policy governing systems with access to PHI.
- Complete this form. Your study contact can help you fill out this form, but general instructions are below.
- Campus: RBHS for most cases
- Sponsor: Your study’s regulatory coordinator.
- Department: Where the study is taking place.
- For NJMS studies, start typing NJMS, then select the speciality.
- For RWJMS studies, start typing RWJMS, then select the specialty.
- After completion, the sponsor will approve the request.
- Rutgers OIT will create your account and you will be emailed your guest NetID.
Step 2: Advarra University Course
- You will need an Advarra University account in order to sign up for the appropriate training modules.
- Submit a request for an Advarra University account by contacting the RBHS Clinical Trials Office via email at clinicaltrials@rbhs.rutgers.edu, or by having your study’s regulatory coordinator make the request on your behalf.
- You can log in to Advarra University anytime at http://university.advarra.com after your account has been created.
After Logging Into Advarra University
- From here, navigate to the Store to see a listing of both free training resources and classroom training available for purchase (they are $0).
- Search for Advarra eReg 1300: Reviewer (Internal or External Monitor) Curriculum and add it to your cart. Complete the checkout (for $0).
- Complete the course.
- Submit your certificate along with the below two forms.
- eReg Access Request
- Requesting Manager: Name of the regulatory coordinator for the study at Rutgers
- User: Your Name
- Change Date: Today’s Date
- Add: Reviewer Access
- Trained: Yes (if training has already been completed)
- eReg Training Record (course completion certificate required)
- Computer-Based Training
- Location – Online
- Instructor Name – N/A (Advarra University)
- Training Title – Advarra eReg 1300: Reviewer (Internal or External Monitor) Curriculum
- Subjects/Documents Covered: Advarra eReg 100: Navigation eLearning & Advarra eReg 110: Using Review Sessions eLearning
- Results: Passed
- Evidence Attached: Yes
- eReg Access Request
Monitors With An Advarra University Account
Step 1: Guest NetID
All monitors are required to have a guest NetID in order to access eReg, due to University policy governing systems with access to PHI.
- Complete this form. Your study contact can help you fill out this form, but general instructions are below.
- Campus: RBHS for most cases
- Sponsor: Your study’s regulatory coordinator.
- Department: Where the study is taking place.
- For NJMS studies, start typing NJMS, then select the speciality.
- For RWJMS studies, start typing RWJMS, then select the specialty.
- After completion, the sponsor will approve the request.
- Rutgers OIT will create your account and you will be emailed your guest NetID.
Step 2: Rutgers Access Forms
If you have previously completed the Monitor training in Advarra University, complete the below two forms, attaching your completed certificate with form 2. If you need to re-download your certificate, login to Advarra University, navigate to the dashboard in the top left, then click completed courses and you will have the option to download the certificate.
-
- eReg Access Request
- Requesting Manager: Name of the regulatory coordinator for the study at Rutgers
- User: Your Name
- Change Date: Today’s Date
- Add: Reviewer Access
- Trained: Yes (if training has already been completed)
- eReg Training Record (course completion certificate required)
- Computer-Based Training
- Location – Online
- Instructor Name – N/A (Advarra University)
- Training Title – Advarra eReg 1300: Reviewer (Internal or External Monitor) Curriculum
- Subjects/Documents Covered: Advarra eReg 100: Navigation eLearning & Advarra eReg 110: Using Review Sessions eLearning
- Results: Passed
- Evidence Attached: Yes
- eReg Access Request
For Rutgers Researchers:
If you do not have an Advarra University Account:
- Submit a request for an Advarra University account by contacting the RBHS Clinical Trials Office via email at clinicaltrials@rbhs.rutgers.edu.
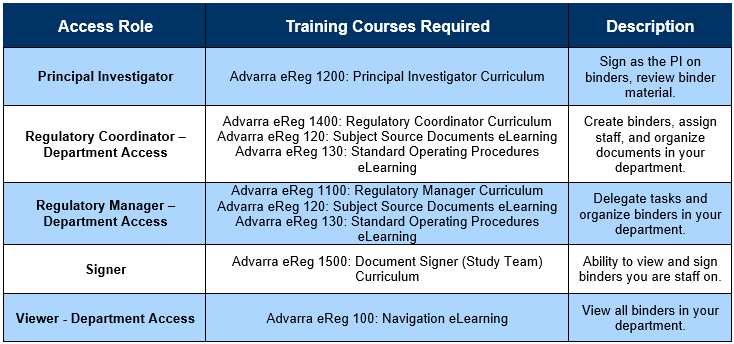
- After creating your account, identify which role you will need by consulting the below chart.
- If you have questions about which role you need, consult your regulatory point of contact, or contact the clinical trials office at clinicaltrials@rbhs.rutgers.edu.
- After identifying your role and once you have an Advarra University account, navigate to Advarra University’s Store (top left) and add the your role’s courses to your cart – they are free!
- After completion of the trainings, submit the following forms:
If you do have an Advarra University Account:
- Identify which role you will need in eReg. If you have questions, consult your regulatory point of contact, or contact the clinical trials office at clinicaltrials@rbhs.rutgers.edu.
- After identifying your role and once you have an Advarra University account, navigate to Advarra University’s Store (top left) and add the your role’s courses to your cart – they are free!
- After completion of the trainings, submit the following forms:
Questions?
Still have questions? Contact the Clinical Trials Office at clinicaltrials@rbhs.rutgers.edu.
